![Tuesday May 26 Objective: Calculate the amount of acid or base needed to neutralize a solution. Checkpoint: – Calculate the [OH-] in a solution that has. - ppt download Tuesday May 26 Objective: Calculate the amount of acid or base needed to neutralize a solution. Checkpoint: – Calculate the [OH-] in a solution that has. - ppt download](https://slideplayer.com/10846268/39/images/slide_1.jpg)
Tuesday May 26 Objective: Calculate the amount of acid or base needed to neutralize a solution. Checkpoint: – Calculate the [OH-] in a solution that has. - ppt download
![Tuesday May 26 Objective: Calculate the amount of acid or base needed to neutralize a solution. Checkpoint: – Calculate the [OH-] in a solution that has. - ppt download Tuesday May 26 Objective: Calculate the amount of acid or base needed to neutralize a solution. Checkpoint: – Calculate the [OH-] in a solution that has. - ppt download](https://images.slideplayer.com/39/10846268/slides/slide_2.jpg)
Tuesday May 26 Objective: Calculate the amount of acid or base needed to neutralize a solution. Checkpoint: – Calculate the [OH-] in a solution that has. - ppt download

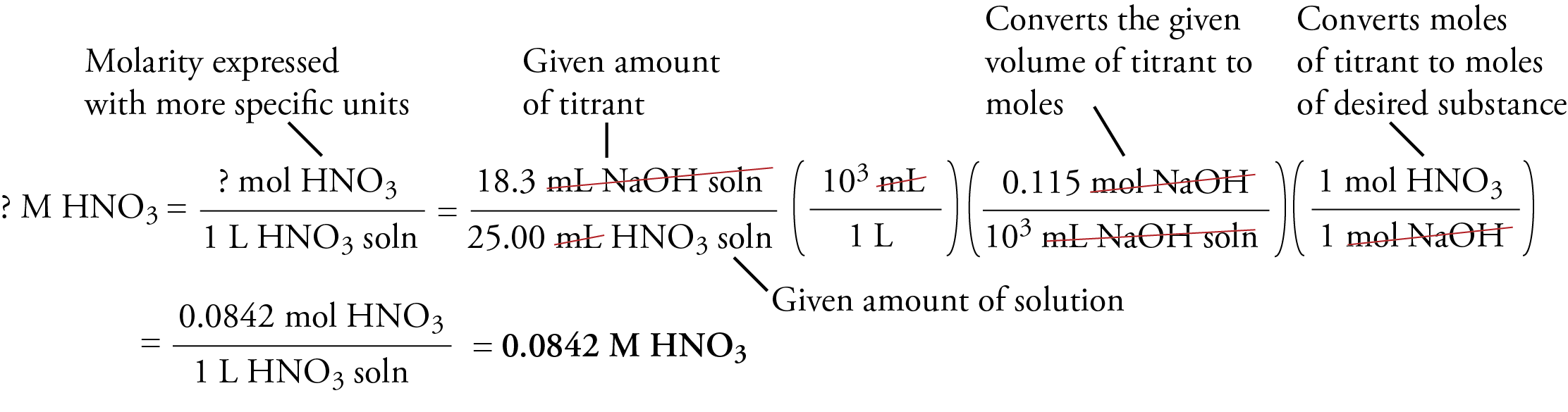
Calculate the concentration of HCl acid if 50 ml of HCl is required to neutralize 25 ml of 1 M NaOH in acid base titration.

Neutralizing Solutions with Sodium Hydroxide | Process & Chemical Formula - Video & Lesson Transcript | Study.com

Question Video: Calculating the Volume of Sulfuric Acid That Completely Neutralizes a Given Volume and Concentration of Sodium Hydroxide | Nagwa

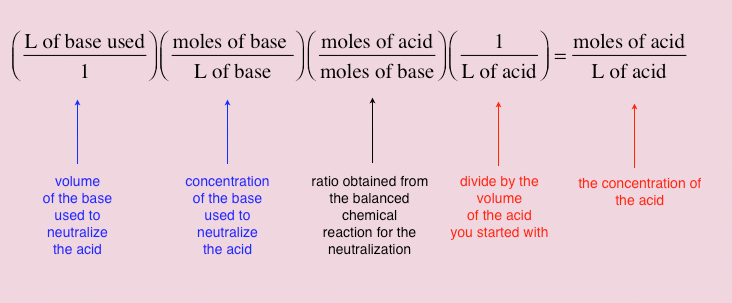
Acid-Base Reactions. Neutralization acid + base salt + water HCl (aq) + NaOH (aq) NaCl (aq) + H 2 O (l) H + + Cl - + Na + + OH - Na + + Cl - + H 2 O (l) - ppt download
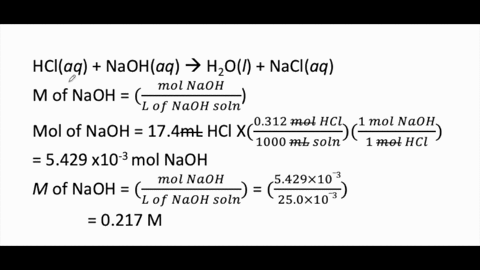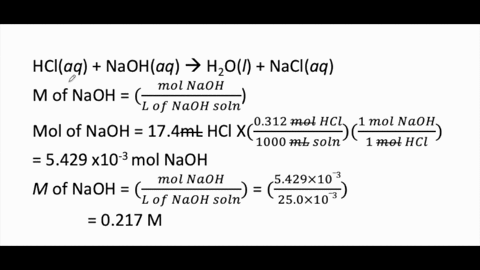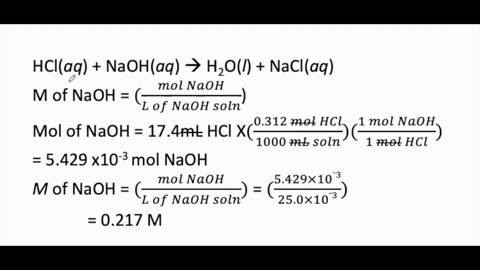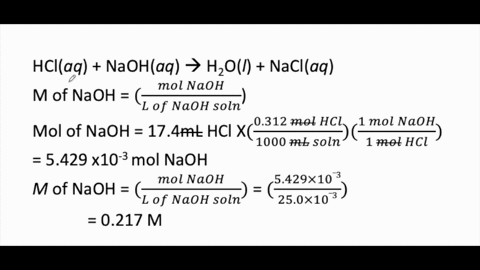





:max_bytes(150000):strip_icc()/168266757-56a131883df78cf772684a99.jpg)