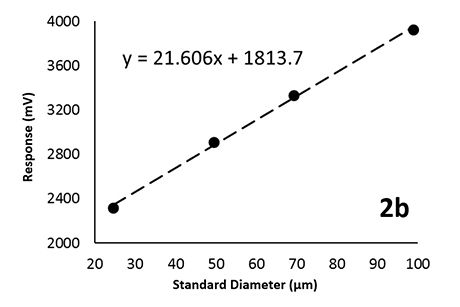
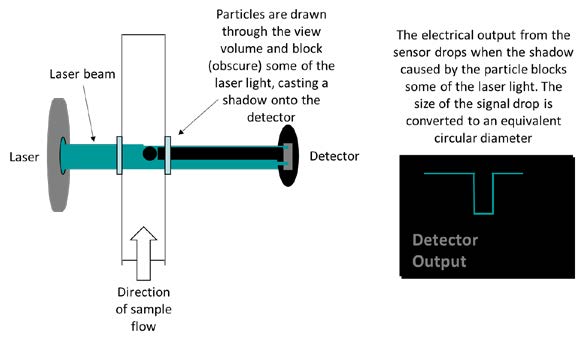
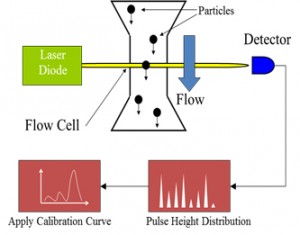
Quantitative Evaluation of Insoluble Particulate Matters in Therapeutic Protein Injections Using Light Obscuration and Flow Imaging Methods - Journal of Pharmaceutical Sciences

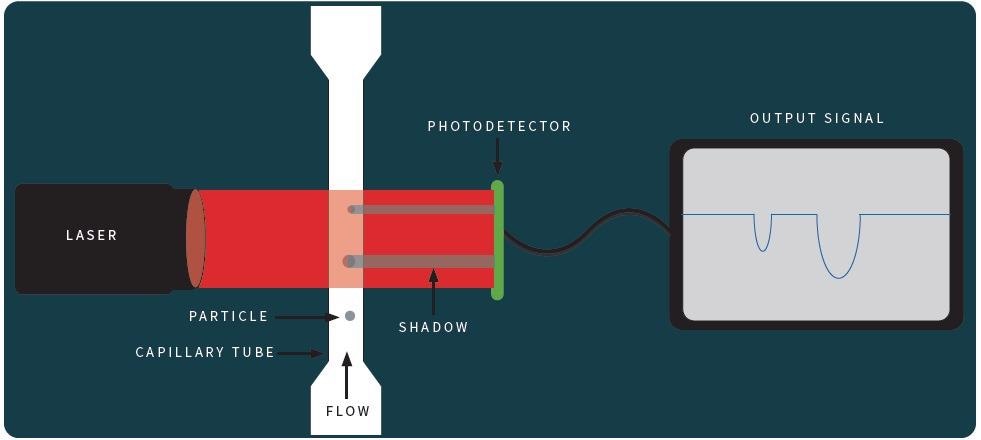
Schematic representation of particle measurement by Light Obscuration. | Download Scientific Diagram

Schematic representation of particle measurement by Light Obscuration. | Download Scientific Diagram

Joe Gecsey Introduction to the new USP 787 Subvisible Particulate Matter in Therapeutic Protein I - YouTube

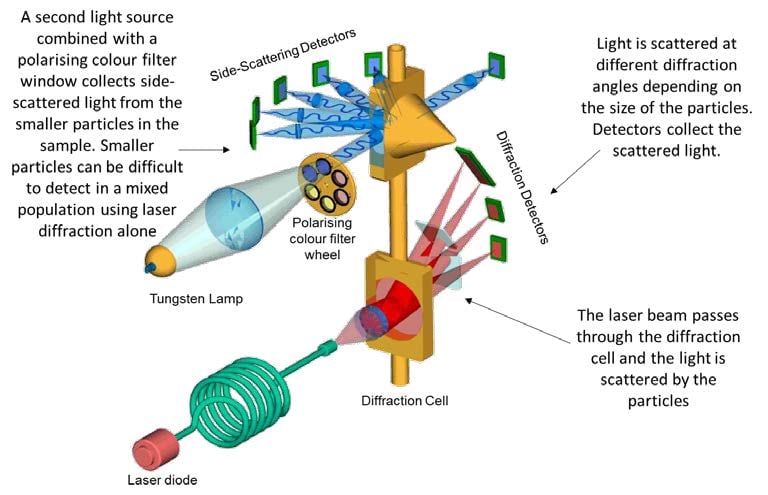
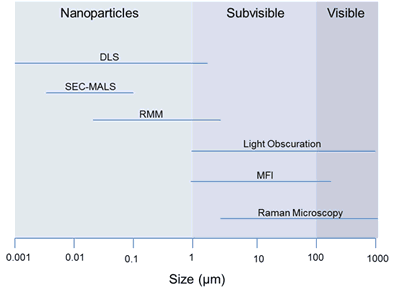
Standards for the Optical Detection of Protein Particles | American Pharmaceutical Review - The Review of American Pharmaceutical Business & Technology


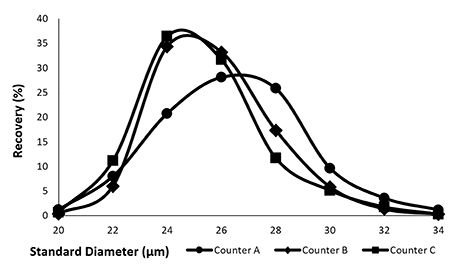
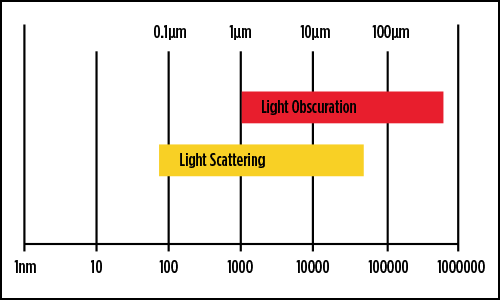
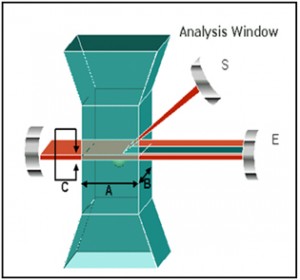
.jpg)