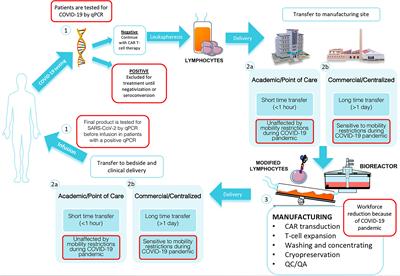
Frontiers | Manufacturing and Management of CAR T-Cell Therapy in “COVID-19's Time”: Central Versus Point of Care Proposals

Optimizing CAR-T Cell Manufacturing Processes during Pivotal Clinical Trials: Molecular Therapy - Methods & Clinical Development
BLA 125646 Tisagenlecleucel 1 FDA Briefing Document Oncologic Drugs Advisory Committee Meeting BLA 125646 Tisagenlecleucel Novar

Tumor response and endogenous immune reactivity after administration of HER2 CAR T cells in a child with metastatic rhabdomyosarcoma | Nature Communications
FDA committee takes on complex gene therapy safety questions with Novartis' Zolgensma providing lessons learned | Fierce Biotech
BLA 125646 Tisagenlecleucel 1 FDA Briefing Document Oncologic Drugs Advisory Committee Meeting BLA 125646 Tisagenlecleucel Novar

Optimizing CAR-T Cell Manufacturing Processes during Pivotal Clinical Trials: Molecular Therapy - Methods & Clinical Development
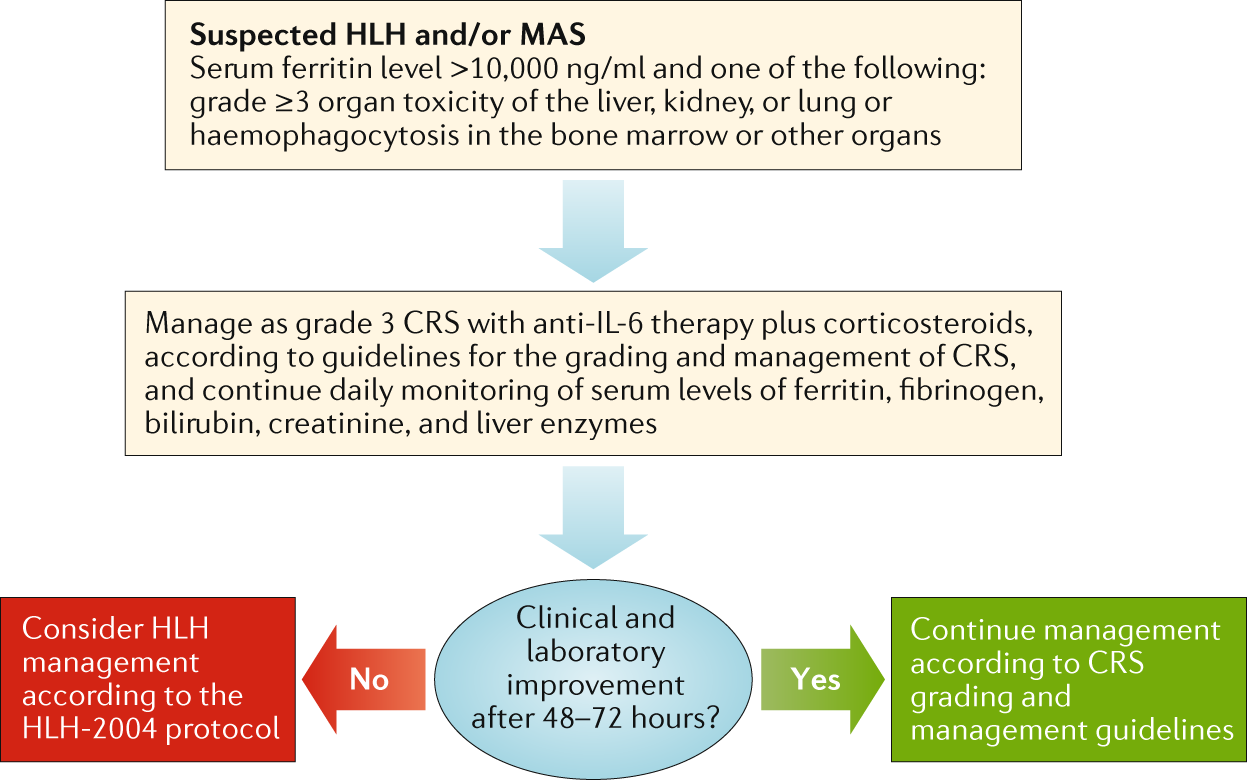
Management guidelines for paediatric patients receiving chimeric antigen receptor T cell therapy | Nature Reviews Clinical Oncology

Optimizing CAR-T Cell Manufacturing Processes during Pivotal Clinical Trials: Molecular Therapy - Methods & Clinical Development
BLA 125646 Tisagenlecleucel 1 FDA Briefing Document Oncologic Drugs Advisory Committee Meeting BLA 125646 Tisagenlecleucel Novar
BLA 125646 Tisagenlecleucel 1 FDA Briefing Document Oncologic Drugs Advisory Committee Meeting BLA 125646 Tisagenlecleucel Novar
ONCOLOGIC DRUGS ADVISORY COMMITTEE BRIEFING DOCUMENT Tisagenlecleucel (CTL019) for the TREATMENT OF PEDIATRIC AND YOUNG ADULT PA

PDF) Chimeric Antigen Receptor-T Cell Therapy: Practical Considerations for Implementation in Europe

FDA Advisory Committee Recommends Approval of CAR-T Cell Therapy and Two New Biosimilars | Biosimilars Law Bulletin