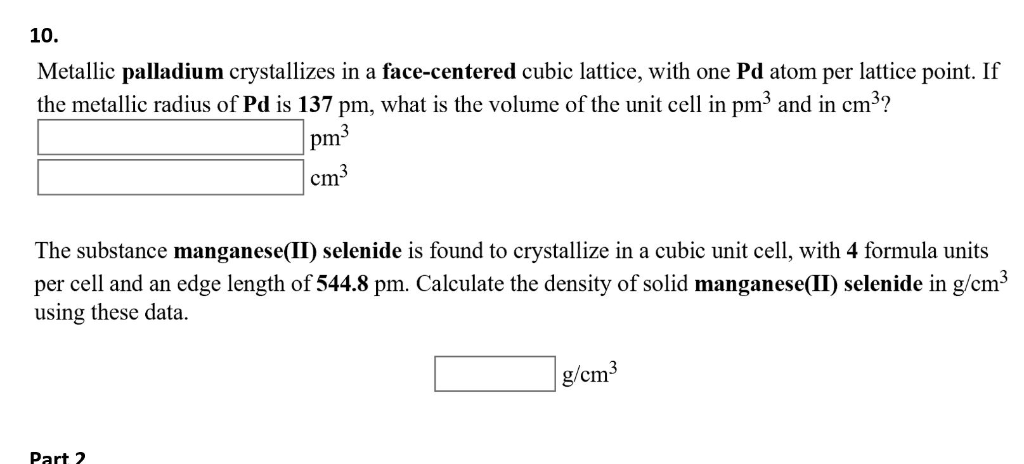
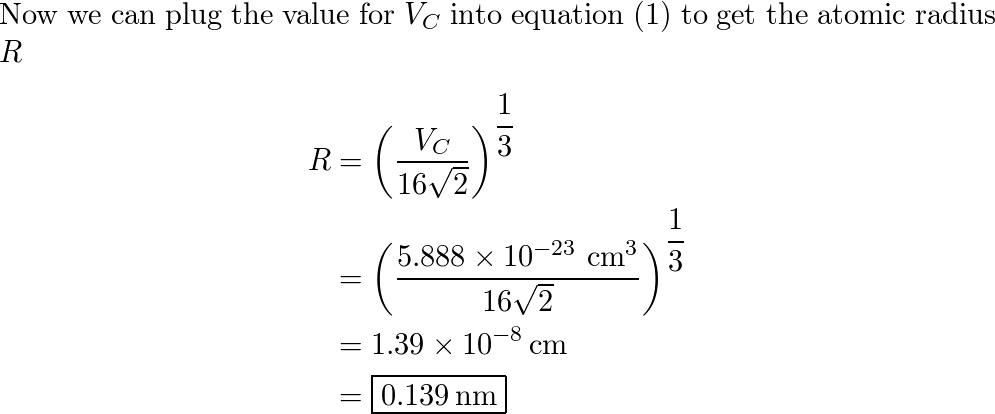Metallic lead crystallizes in a face-centred cubic lattice, with one Pb atom per lattice point. If the metallic radius of Pb is 175 pm, what is the volume of the unit cell
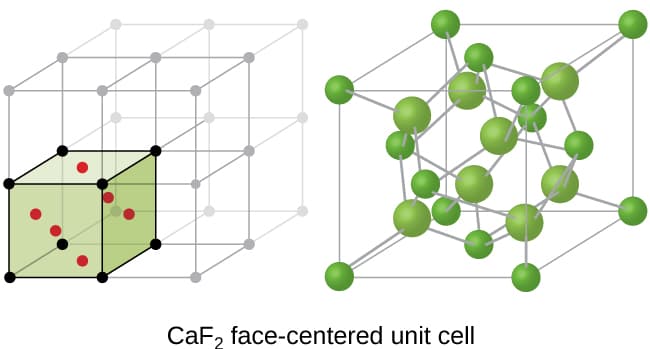
A mineral having the formula AB2 crystallizes in the cubic closepacked lattice with the A atoms occupying the lattice points What are the coordination numbers of the A and B atoms

OneClass: A metal crystallizes in the face-centered cubic (FCC) lattice. The density of the metal is ...

The metal palladium crystallizes in a face-centered cubic lattice with an edge length of 388.8 pm. What is the density of the palladium? | Homework.Study.com

Gold occurs as face centred cube and it has a density of 19.30 kg dm ^-3 .Calculate atomic radius of gold. (Molar mass of Au = 197 )

OneClass: Palladium crystallizes with a face-centered cubic structure. It hasa density of 12.0 g/cm3,...
An element crystallizes in an FCC lattice and the edge of the unit cell is 0.559nm. The density is 3.19g/cm. What is the atomic weight? - Quora
PLEASE HELP! 80 points!! A metal crystallizes in the face‑centered cubic (FCC) lattice. The density of the - Brainly.com

SOLVED:Palladium crystallizes with a face-centered cubic structure. It has a density of 12.0 g/cm3, a radius of 138 pm, and a molar mass of 106.42 g/mol. Use these data to calculate Avogadro's

A metal crystallizes in the face-centered cubic unit cell with an edge length of 320 pm. \\ A. What is the radius of the metal atom? B. The density of the metal

Problem.docx - Problem #1: Palladium crystallizes in a face-centered cubic unit cell. Its density is 12.023 g/cm3. Calculate the atomic radius | Course Hero

Gold occurs as face centred cube and it has a density of 19.30 kg dm ^-3 .Calculate atomic radius of gold. (Molar mass of Au = 197 )

SOLVED: Rhodium has a density of 12.41 g>cm3 and crystallizes with the face-centered cubic unit cell. Calculate the radius of a rhodium atom.
Metallic gold crystallizes in the facecentred cubic lattice. The length of the cubic unit cell is a = 4.070 Å. - Sarthaks eConnect | Largest Online Education Community

Interactions between Hydrogen and Palladium Nanoparticles: Resolving Adsorption and Absorption Contributions - Moumaneix - 2023 - ChemElectroChem - Wiley Online Library

roblem.docx - problem #1: Palladium crystallizes in a face-centered cubic unit cell. Its density is 12.023 g/cm3. Calculate the atomic radius of | Course Hero