Puma Biotechnology and Pierre Fabre Enter into Exclusive License Agreement to Develop and Commercialize NERLYNX® (neratinib) in Europe | Business Wire

Is Puma off the cancer M&A list? With Nerlyx marketing deal, some investors think so | Fierce Pharma
A Top Puma Bio Exec Resigns Ahead of FDA Panel, Analysts Defend With Conflicting Stories - TheStreet

Pint-Pharma - Puma Biotechnology and Pint Pharma Enter into Exclusive Licensing Agreement to Commercialize NERLYNX® (neratinib) in Latin America
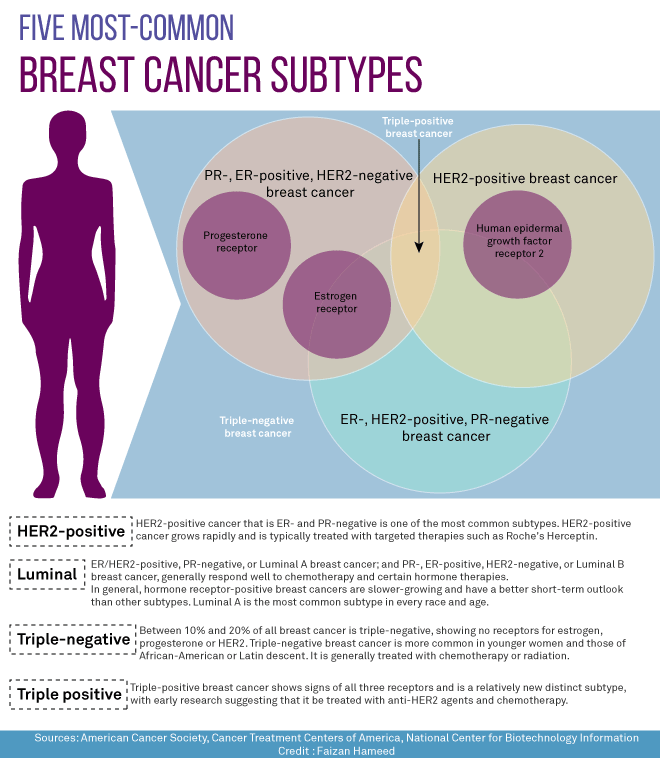
Efficacy of neratinib in hormone receptor-positive patients who initiated treatment within 1 year of completing trastuzumab- bas

Puma Biotechnology, Pierre Fabre enter into license agreement to develop and commercialize NERLYNX in Europe - Pharmaceutical Business review

Puma Biotechnology obtains dismissal of opposition against its European patent relating to treatment for gefitinib/erlotinib-resistant cancers and cancers with T790M EGFR mutation | Experience | Jones Day