USFDA: Teva Pharmaceutical recalls 38,858 bottles of drugs made by Emcure Pharmaceuticals in US market - The Economic Times

Teva USA Recalls Fentanyl Buccal Tablets Manufactured and Labeled for Mayne Pharma Inc. - Spine Intervention Society

Teva's recall of U.S.-made drugs latest example of contamination fears in generic marketplace; report says Lilly, Pfizer and former Mylan plant in Morgantown have been cited in the past | WV News
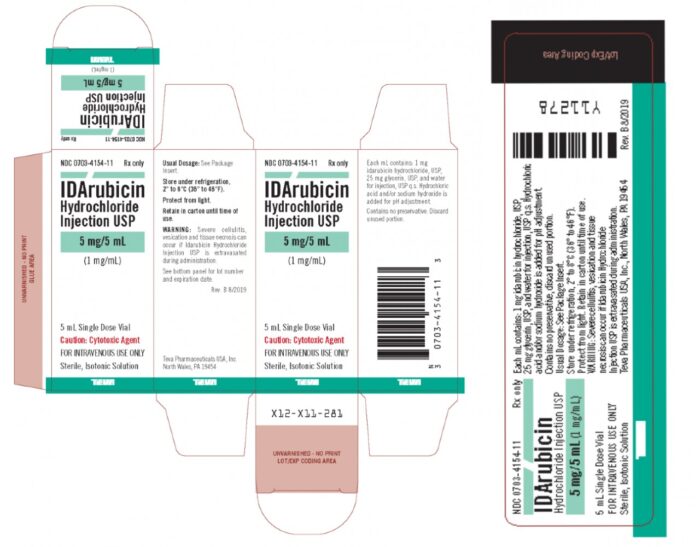
Teva Pharmaceuticals Recalls Acute Myeloid Leukemia Drug Over Particulate Matter Contamination - Top Class Actions