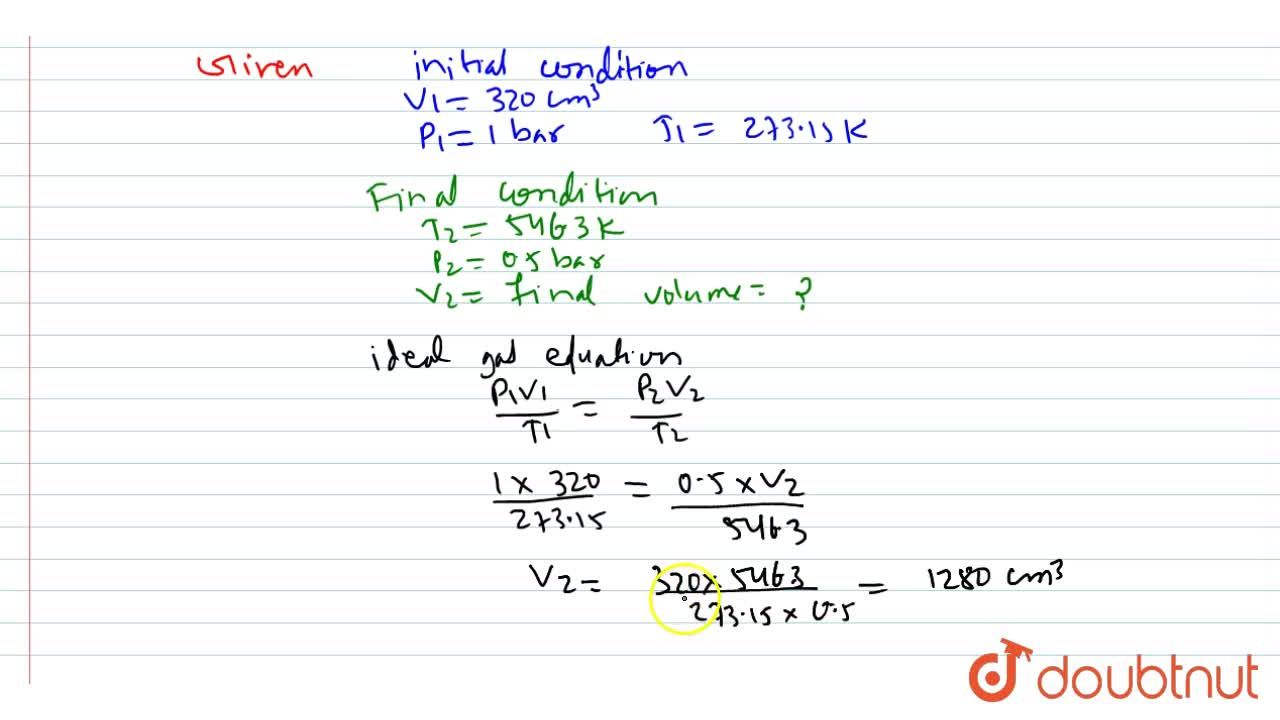
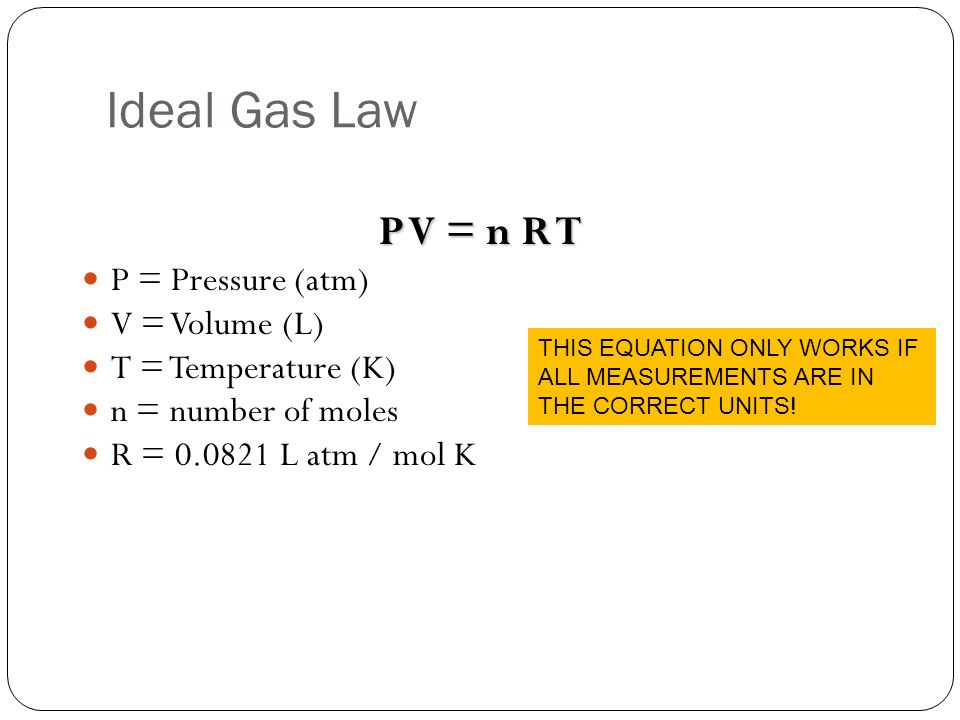
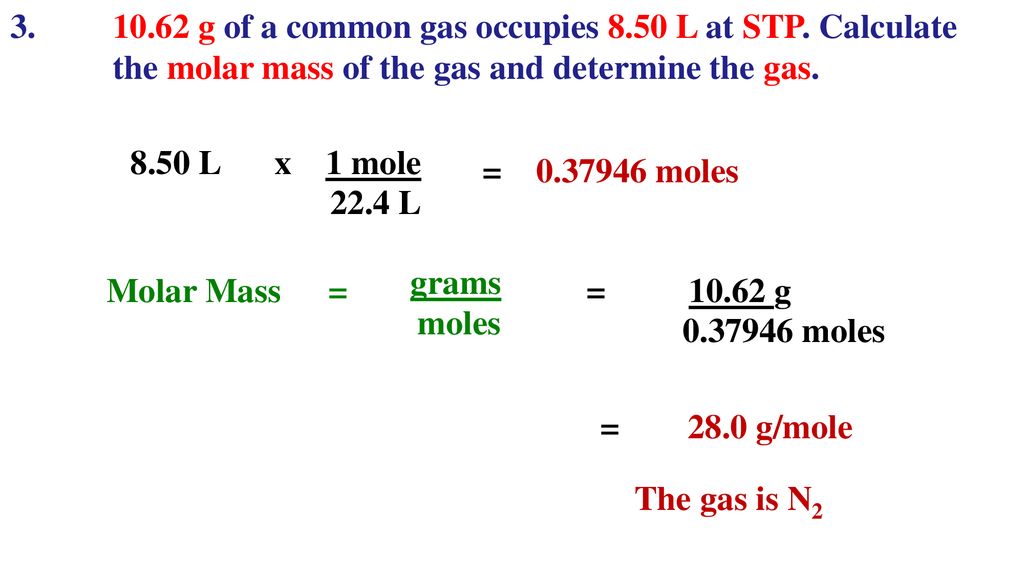
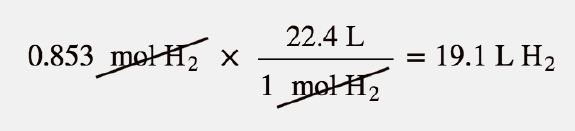![Calculate the volume occupied by 0.1 mole of CO2 at STP?[Please give your answer in the form of nearest integer] Calculate the volume occupied by 0.1 mole of CO2 at STP?[Please give your answer in the form of nearest integer]](https://i.ytimg.com/vi/GKCtgQzOKVk/maxresdefault.jpg)
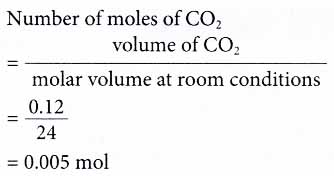
Calculate the volume occupied by 0.1 mole of CO2 at STP?[Please give your answer in the form of nearest integer]

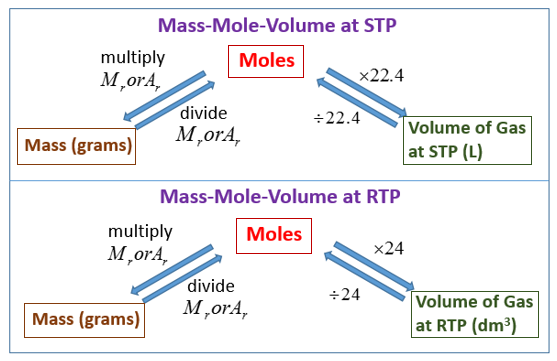
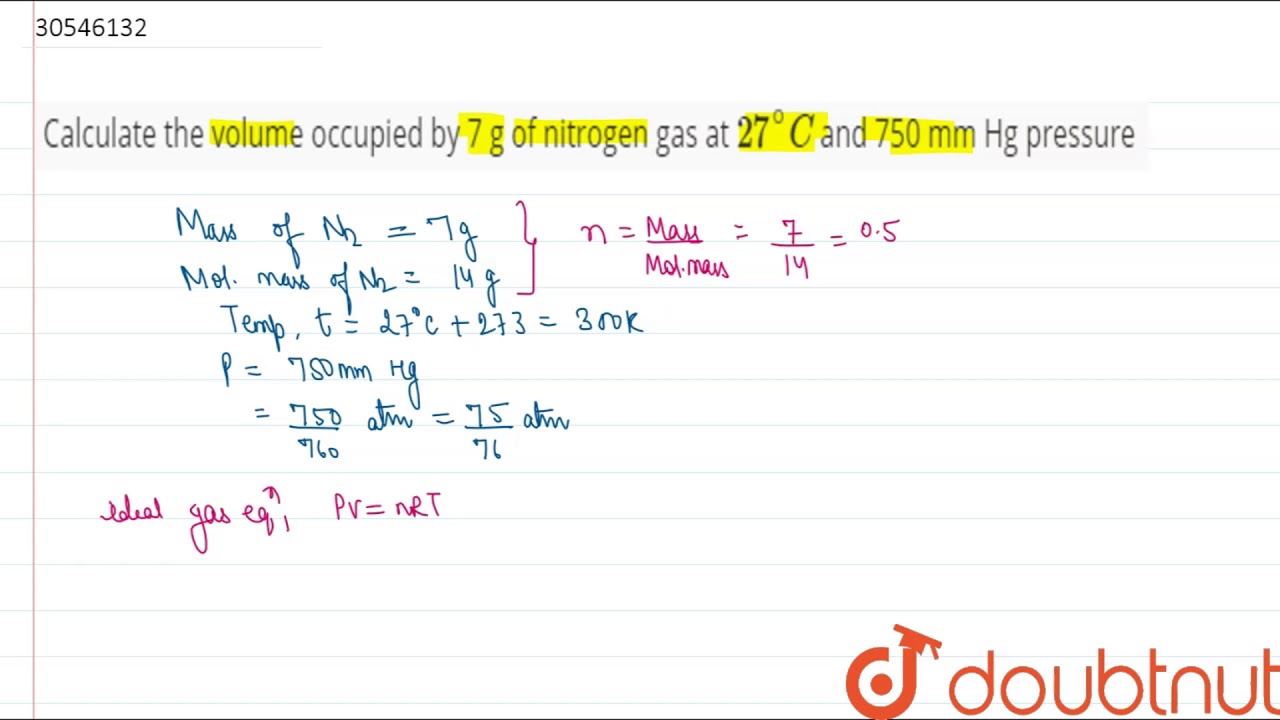
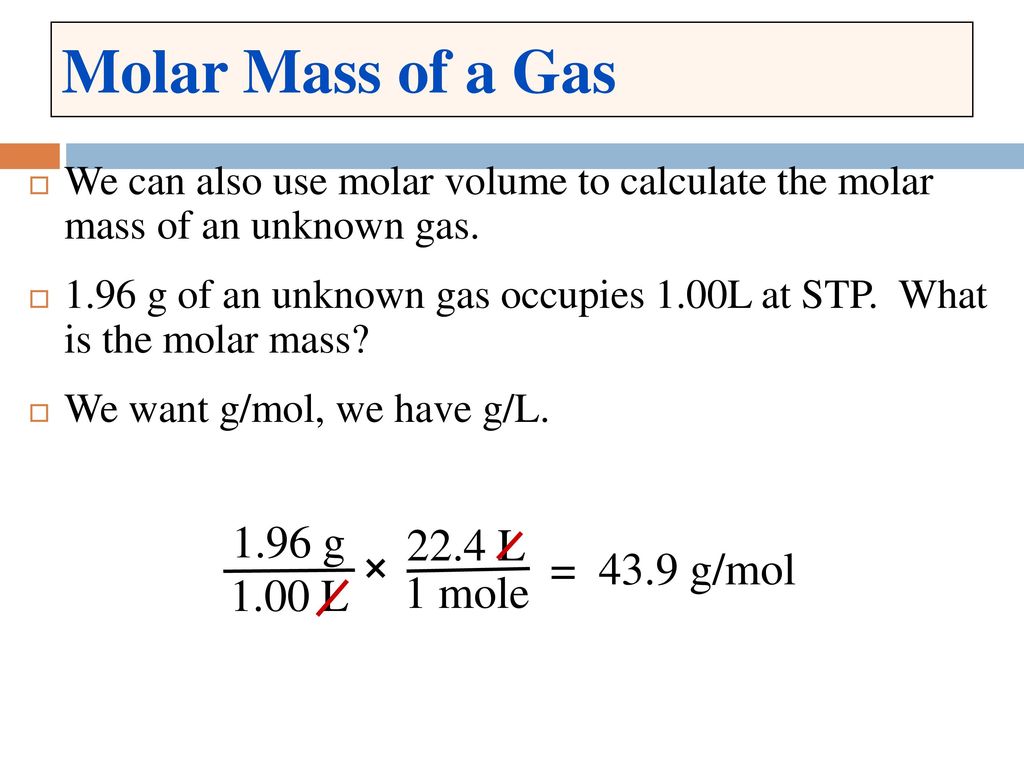
SOLVED:Use molar volume to calculate each of the following at STP: a. the volume, in liters, of 6.40 \mathrm{g} of \mathrm{O}_{2} gas b. the number of grams of \mathrm{H}_{2} in 1620 \mathrm{mL}